APC RESISTANCE UNIVERSITY
Introduction | Biochemistry | Clinical Aspects | Key Reports | PubMed Abstracts | References | Assay Methods | APC Resistance Phenotyping | Factor V Mutation Screening | FAQ
CLINICAL ASPECTS
Hypercoagulable state and thrombophilia
A hypercoagulable state is a condition that favours coagulation, as recognized by increased thrombin generation. Hypercoagulability can be due to a number of factors, which can either be inherited (primary) or acquired (secondary) (Table 2).
Thrombophilia is the clinical term for a hypercoagulable state that causes an increased tendency to thrombosis. Several genes have been implicated with inherited thrombophilia, although only factor V (APC resistance), antithrombin, protein C and protein S have been clearly linked to an increased risk of venous thromboembolism (Table 3).7,50 Of these, APC resistance is the most common, both among patients and in the general population (Table 4).9-12
Diagnosing APC resistance
The development of a simple, APTT-based assay that measures the anticoagulant response in plasma to added purified APC, facilitated the characterization of the APC resistance phenotype.8 In the classic test kit, Coatest® APC™ Resistance, two APTT reactions are performed, one in the presence of a carefully-defined quantity of APC and the other in its absence.51 The relationship between the two clotting times is expressed as a ratio, called the APC ratio. Healthy individuals have an APC ratio in the range 2-5, whereas APC-resistant individuals are recognized by an APC ratio below or equal to about 2. The precise cut-off for a diagnosis may vary slightly depending on the instrument type used as well as the individual condition of the instrument.226-228
Table 4. Prevalences of inherited thrombophilia in various populations. Prevalences are estimates based on references.4,47,50 VT= venous thrombosis
| Genetic defect | General population | All VT cases | Familial VT cases | No. of mutations |
| APC resistance | 5% | 20% | 50% | 1 |
| Antithrombin deficiency | 0.1% | 1% | 4% | >79 |
| Protein C deficiency | 0.2% | 3% | 5% | >160 |
| Protein S deficiency | n.d. | 2% | 5% | >40 |
The phenotypic APC ratio reflects the severity of the hypercoagulable state and provides information on the thrombotic risk associated with inherited and possibly acquired APC resistance. A modified APC resistance test, Coatest® APC™ Resistance 5, is available which exclusively detects factor V-related APC resistance, i.e. APC resistance due to the FV:Q506 mutation.52 The assay modification involves a predilution of plasma samples with an excess of stabilized factor V-deficient plasma (V-DEF Plasma) containing a heparin antagonist. Since the predilution with VDEF Plasma normalizes the basal APTT reaction, it safely allows for APC resistance-testing of plasma from patients on oral anticoagulant or heparin therapy. It also produces a complete discrimination for FV:Q506, which makes the modified assay highly suitable for factor V mutation screening. The two APTT-reactions obtained from the modified APC resistance assay are expressed as an APC-V ratio, calculated in the same way as the APC ratio obtained from the classic test. The APC-V ratio provides genotypic information concerning factor V and is generally lower than the APC ratio for the same sample, regardless of the instrument used. Typical APC-V ratio ranges for different factor V genotypes are 2.2 – 3.2 for normal FV:R506, 1.4 – 1.8 for heterozygous FV:Q506, and 1.1 – 1.3 for homozygous FV:Q506.
World distribution of APC resistance and FV:Q506
An overall cumulative analysis of different patient groups with venous thromboembolism shows that the prevalence of APC resistance is about 20% (range 0-64%, see Tables 5 and 6).9-12,53-69 The variation in the prevalence of APC resistance between clinical studies are related mainly to differences in selection criteria and the uneven distribution of the FV:Q506 allele in the general population in different parts of the world (Figure 8). The highest prevalences of APC resistance and the FV:Q506 mutation have been found among healthy controls in several European populations of Caucasian origin, most notably in Cypriot Greek (13%),77 Swedish (11%),78 French (10%),75 British (9%),77 German (9%)72 and Dutch (5%)10 people. In contrast, the mutation appears to be rare among Chinese70,77,83 and absent among Japanese60,76,79 and Africans (Negroid).77,92 This could account for the relatively low incidence of venous thromboembolism reported these ethnic populations.71,77 The average FV:Q506 carrier frequency among healthy European controls is about 5%. Approximately 0.1% of a Caucasian population can be expected to be homozygous for this mutation.
Distribution of the FV:Q506 mutation in the world population

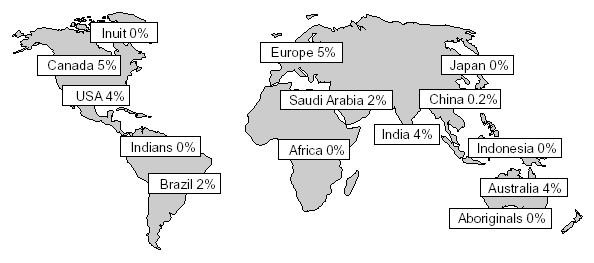
1) FV:Q506 mutation determined using a DNA-based assay.
| Country / region | FV:Q506 Ref. | n/n test. | % |
| Australia, Aboriginal | 0/73 | 0 | 77 |
| Brazil, Indians | 0/83 | 0 | 81 |
| Brazil, Blacks | 1/137 | 0.7 | 81 |
| Brazil | 2/100 | 2 | 57 |
| Canada | 19/356 | 5.3 | 82 |
| China, Han | 1/618 | 0.2 | 83 |
| China, Hong Kong | 0/293 | 0 | 70 |
| Finland | 4/137 | 2.9 | 93 |
| France, Paris | 5/229 | 2.2 | 74 |
| France, Strasbourg | 17/176 | 9.7 | 75 |
| Germany, South | 14/180 | 7.8 | 85 |
| Germany, North-East | 58/814 | 7.1 | 86 |
| Germany, North-West | 18/190 | 9.5 | 72 |
| Greece | 17/203 | 8.4 | 80 |
| Greek Cypriots | 25/187 | 13 | 77 |
| Greenland, Inuit | 0/133 | 0 | 87 |
| Iceland | 3/96 | 3.1 | 77 |
| India, North | 3/70 | 4.3 | 88 |
| Indonesia, Sumatra | 0/105 | 0 | 77 |
| Italy | 9/344 | 2.6 | 89 |
| Japan | 0/192 | 0 | 76 |
| Jamaica | 0/91 | 0 | 77 |
| Kenya | 0/60 | 0 | 77 |
| Mongolians | 0/36 | 0 | 77 |
| Netherlands | 14/474 | 3.0 | 54 |
| Papua New Guinea | 0/95 | 0 | 77 |
| Peru, Indians | 0/19 | 0 | 77 |
| Saudi Arabia | 5/200 | 2.5 | 90 |
| Senegal | 0/96 | 0 | 77 |
| Sweden | 11/101 | 11 | 78 |
| Taiwan, Aboriginals | 0/83 | 0 | 77 |
| United Kingdom | 21/237 | 8.9 | 77 |
| United Kingdom | 5/144 | 3.5 | 73 |
| USA, Blacks | 3/214 | 1.4 | 91 |
| USA | 42/704 | 6.0 | 69 |
| Zambia | 0/95 | 0 | 77 |
2) APC resistance phenotype determined using the classic APC resistance test.
| Country | APC resistant n/n test. | % | ref. |
| Austria | 1/50 | 2 | 55 |
| France | 1/75 | 1.3 | 12 |
| France | 2/50 | 4 | 56 |
| Italy | 20/1212 | 1.2 | 64* |
| Japan | 3/291 | 1.0 | 59 |
| Netherlands | 14/301 | 4.6 | 10 |
| Poland | 1/110 | 0.9 | 62* |
| Spain | 3/107 | 2.8 | 65 |
| Sweden | 9/130 | 6.9 | 11 |
| USA | 2/39 | 5.1 | 67 |
* abstract
3) APC resistance due to FV:Q506 in USA determined using the modified APC resistance test.
| USA ethnic origin | US pop. millions | % FV:Q506 mutation |
| Caucasians | 185 | 5.3 |
| Hispanic | 25.8 | 2.2 |
| Afroamericans | 32.7 | 1.4 |
| Native Indians | 2.1 | 1.3 |
| Asians | 7.5 | 0.46 |
Data was presented by Dr. J Miletich at the 42nd ISTH Subcommitte Meeting in Barcelona in June 1996. In total 2242 plasma samples were investigated from individuals of different ethnic origin representing a typical US population.
Figure 8. Reported prevalences of APC resistance and the factor V:Q506 mutation in the global population.
The origin of the FV:Q506 mutation
Several investigators have suggested that the high prevalence of the FV:Q506 mutation could be due to the evolutionary advantage it would confer, which has helped to maintain and spread the mutation.71,77,81 It is possible that the selective disadvantage of a life-long hypercoagulable state could be balanced by, for example, the protection against excessive blood loss during delivery and menstruation. The selective risk of the FV:Q506 mutation would also be of less historical importance as people in ancient times were not exposed to modern risk factors for thrombosis (e.g. oral contraceptives, surgery, sedentary lifestyle etc.). The high allelic frequency of FV:Q506 in Caucasian populations and its linkage to different polymorphisms in the factor V gene, supports the hypothesis that the mutation occurred as a single event in the ancient European population.13,74-75,94 The time of this event would be approximately 30,000 years ago, i.e. after the diversion of Africans from non-Africans (140,000 years ago) and after the diversion of Caucasians from Mongolic populations (70,000 years ago), but before the diversion of Caucasian subpopulations. However, since the FV:Q506 mutation involves a CpG dinucleotide, which is an established hotspot for mutation, the possibility of recurrent mutations in other races should not be ruled out altogether.88
Clinical manifestations of APC resistance
The clinical manifestations of inherited, heterozygous protein defects in familial thrombophilia involving antithrombin, protein C, protein S and factor V (APC resistance) are fairly similar. Mutations affecting the qualitative or quantitative function of these proteins often result in venous thromboembolism at a young age (before the age of 45 years) and are followed by a tendency towards recurrent thrombotic episodes.50 The most common manifestation of APC resistance is deep venous thrombosis (DVT) of the lower limbs, with or without pulmonary embolism, which accounts for about 90% of all thrombotic episodes.50,53,96 Other, less frequent, manifestations include superficial thrombophlebitis96 and unusual sites for thrombosis such as the mesenteric,96 central retinal,97-101 portal,102,103 internal jugular,104 and cerebral veins.105,106
Thrombotic risk
The relative risk of DVT for carriers of the FV:Q506 mutation compared to non-carriers has been estimated to increase eight-fold for heterozygotes (single defect) and 80-fold for homozygotes (double defect).54 Since aging itself is a risk factor for thrombosis, the absolute risk increases with age.69 A similarly increased risk of pulmonary embolism has been observed by some investigators,216 although this could not be confirmed by others.217,218 The risk of recurrent venous thromboembolism in carriers of the FV:Q506 mutation has been reported to be three to fivefold higher compared to patients without the mutation,223,286 but again there is no consensus on this matter.224
FV:Q506 is a mild risk factor by itself
The penetration of clinical manifestations among APC resistant individuals is variable, and a majority of heterozygous carriers of FV:Q506 actually never experience any symptoms. In fact, not even homozygous carriers will necessarily be affected by thrombosis during their lifetime.43 These facts illustrate that FV:Q506 is a mild risk factor per se and that the probability of APC-resistant individuals developing thrombosis is dependent on the coexistence of other risk factors. About 60% of APC-resistant patients have their first thrombotic event in combination with pregnancy, oral contraceptives, trauma or surgery.16,53,96 Furthermore, because of the high prevalence of APC resistance in the general population, its combination with other genetic defects is not unusual. A wide range of disorders have been reported in connection with APC resistance, implicating its part in the development of thrombotic complications. These include the Budd-Chiari syndrome,107-109 nephrotic syndrome,110 leg ulcers,111,112 heparin-induced thrombocytopenia,113 priapism,114polycythemia vera,115 essential thrombocythemia,115 child-thrombosis,116-119 cutaneous skin necrosis,120,121 neonatal purpura fulminans,165 acute lymphoblastic leukemia,166 systemic sclerosis,170 and preeclampsia.282
Table 5. Prevalence of APC resistance phenotype in patients with venous thrombosis.
| Country | Venous thrombosis n pts. | Venous thrombosis n APC res. | References |
|---|---|---|---|
| Austria | 40 | 7 (17%) | Halbmayer et al55 |
| France | 175 | 29 (17%) | Trossaërt et al56 |
| France | 48 | 9 (19%) | Cadroy et al12 |
| France | 183 | 24 (13%) | Samaha et al53 |
| Italy | 20 | 2 (10%) | *Tosetto et al64 |
| Italy | 118 | 33 (28%) | De Stefano et al68 |
| India | 28 | 6 (21%) | Pati et al63 |
| Japan | 43 | 5 (12%) | Kambayashi et al59 |
| Japan | 22 | 4 (18%) | Fujimura et al60 |
| Netherlands | 301 | 64 (21%) | Koster et al10 |
| Poland | 72 | 9 (12%) | *Lopaciuk et al62 |
| Sweden | 104 | 34 (33%) | Svensson et al11 |
| Spain | 72 | 3 (4%) | *Borell et al65 |
| Spain | 176 | 14 (8%) | Ortega et al61 |
| USA | 25 | 16 (64%) | Griffin et al9 |
| USA | 37 | 9 (24%) | Chusman et al67 |
| Total | 1,464 | 268 (18%) |
* abstract paper
Table 6. Prevalence of the FV:Q506 mutation in patients with venous thrombosis according to gene analysis.
| Country | Venous thrombosis n pts. | Venous thrombosis n FV:Q506 | References |
|---|---|---|---|
| USA | 121 | 14 (11%) | Ridker et al69 |
| Japan | 22 | 0 (0%) | Fujimura et al60 |
| France | 87 | 14 (16%) | Alhenc-Gelas et al66 |
| Netherlands | 301 | 53 (17%) | Bertina et al13 |
| Netherlands | 27 | 10 (37%) | Voorberg et al14 |
| Netherlands | 471 | 92 (19%) | Rosendaal et al54 |
| Brazil | 40 | 8 (20%) | Arruda et al57 |
| Australia | 45 | 12 (26%) | Ma et al58 |
| Total | 1,114 | 203 (18%) |
* abstract paper
Table 7. Prevalence of the FV:Q506 mutation in patients with arterial thrombosis compared with controls according to factor V gene analysis.
| Country N pts. | Arterial thrombosis n pts. | Arterial thrombosis n FV:Q506 | Controls | References |
|---|---|---|---|---|
| Australia | 222 | 11 (5.0%) | (4%) | van Bockxmeer et al129 |
| Finland | 358 | 16 (4.5%) | (2.9%) | Kontula et al93 |
| Germany | 224 | 21 (9.4%) | (4.1%) | März et al132 |
| Sweden | 101 | 18 (18%) | (11%) | Holm et al78 |
| UK | 386 | 16 (4.1%) | (5.6%) | Catto et al133 |
| USA | 583 | 32 (5.5%) | (5.5%) | Ridker et al69 |
Arterial thromboembolism
Although there is a clear link between APC resistance due to the FV:Q506 mutation and venous thrombosis, the same link to arterial thrombosis is enigmatic. Several studies have reported the presence of APC resistance in young stroke patients, suggesting that it contributes to the pathophysiology.55,122-126,163 Halbmayer et al for example, found that 20% (6 out of 30) of young Austrian stroke patients were APC-resistant according to the classic APC resistance test.55 In contrast, other studies of stroke patients showed no increased prevalence of either the APC resistance phenotype or FV:Q506 mutation compared to healthy controls (Table 7).67,69,93,133-135,169
The possible correlation between the FV:Q506 mutation and the risk of ischemic heart disease, particularly myocardial infarction, has also been investigated. With the exception of two papers,78,132 the general conclusion is that the FV:Q506 mutation is not an important risk factor for arterial thrombosis in heterozygotes,67,69,72,75,93,127-131,164 but may have a role in homozygotes.126,137 Some papers have appeared recently which may help to clarify the situation. These suggest that acquired (or inherited) APC resistance, independent of the FV:Q506 mutation, may indeed be an important risk factor for arterial thrombosis.139-141 These observations are most interesting and they call for an evaluation in different patient groups, using the classic APC resistance test, to explore whether the APC ratio may be predictive for both venous and arterial thrombotic events.
APC ratios in thrombophilic families
Zöller et al investigated 50 thrombosis-prone Swedish families with APC resistance (Figure 9).16 In three of these families the FV:Q506 mutation was not present, suggesting another, as yet unidentified, cause of APC resistance. In total, 308 family members were investigated; 146 normal, 144 heterozygotes and 18 homozygotes. APC ratios were low in all the homozygous and most of the heterozygous cases. APC ratios in the APC-resistant individuals who lacked the mutation ranged from 1.3 to 2.0. Heterozygotes with a history of thrombosis had significantly lower APC ratios than those without thrombosis and none of the heterozygotes with APC ratios >2.0 had experienced thrombosis. Moreover, relatives without the mutation but with thrombotic histories had on average lower APC ratios than those without thrombosis. Significant differences in thrombosis-free survival curves and APC ratios were observed between the groups (Figure 10), thus confirming that APC resistance is an important risk factor for thrombosis. By the age of 33, 8% of the normals, 20% of the heterozygotes and 40% of homozygotes had experienced manifestations of venous thrombosis. The average age for the first thrombotic event was 25 (range 10 to 40 years) for homozygotes and 36 (range 18 to 71 years) for heterozygotes. In the thrombosis-prone families the observed incidence of thrombosis was higher than expected, suggesting that these families have been affected by additional genetic defects.
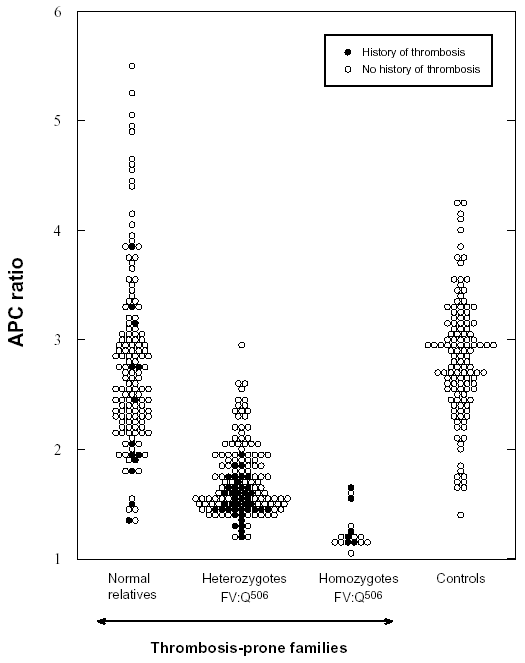
Figure 9. Relationship between APC ratios and the FV:Q506 allele in families with APC resistance.
APC ratios in non-anticoagulated carriers of the FV:Q506 mutation and in family members without the mutation (normals), compared with unrelated healthy controls. The APC ratios were determined by the original APC resistance test method. Using a cut-off value of 2.0, the sensitivity and specificity for the FV:Q506 allele would be 85% and 87%, respectively. APCratios (mean ±SD) in normals 2.8 ±0.8, heterozygotes 1.7 ±0.3, homozygotes 1.3 ±0.2, and controls 2.8 ±0.6. Reproduced by permission of Zöller et al and The American Society for Clinical Investigation.16
Figure 10. Thrombosis-free survival curves for different FV:Q506 genotypes.
Multiple genetic defects
The underlying cause of familial thrombophilia has long been considered to be single-gene defects. However, the notion that this idea was too simple has been reported repeatedly in recent years, particularly in connection with protein C deficiency.142-143 It was found that the same type of mutation could affect different families differently, giving rise to the idea that several genetic risk factors in combination are usually needed for clinical manifestations to occur. Strong evidence supporting this view came with the discovery of APC resistance. Its high prevalence in the general population and the observation that individuals with combinations of inherited risk factors (e.g. FV:Q506 and protein C deficiency) suffer more severely from thrombosis, and at a younger age, than those with single defects, has led to the idea of familial thrombophilia being primarily a polygenetic syndrome.47,144-145,168 Most studies have confirmed this by showing a relatively high incidence of FV:Q506 among clinically symptomatic probands in thrombophilic families with protein C,146-147 protein S,148-149,167 or antithrombin deficiency (Table 8).150-151 The observed frequency variation of the FV:Q506 mutation among these probands is probably related to population differences.152-153 Other interesting candidates for polygenetic familial thrombophilia involving FV:Q506, include hyperhomocysteinemia (due to either cystathione-b-synthase or methylenetetrahydrofolate reductase deficiency),154-155 familial antiphospholipid syndrome,156 heparin cofactor II deficiency,157 plasminogen deficiency,158 and possibly also elevated prothrombin levels due to a 20210 AG genotype in the prothrombin gene.281
Table 8. The incidence of thrombotic episodes in thrombophilic families, related to the number of genetic defects (‘hits’).
| One hit | One hit | Two hits | Ref. |
|---|---|---|---|
| 20%FV:Q506 | 54% AT def. | 92% FV:Q506 + AT def. | 150 |
| 13% FV:Q506 | 31% PC def. | 73% FV:Q506 + PC def. | 147 |
| 19% FV:Q506 | 19% PS def. | 72% FV:Q506 + PS def. | 148 |
AT = antithrombin, PC = protein C, PS = protein S
Influence of FV:Q506 in hereditary bleeding disorders
The influence of FV:Q506 on inherited bleeding disorders has also been studied. A possible moderation of the hemophilia A (factor VIII deficiency) phenotype has been observed in some cases,159 although this was not seen in others.160 A more surprising influence of FV:Q506 is seen in cases of parahemophilia (factor V deficiency). Heterozygotes for this rare bleeding disorder are often asymptomatic, since they have one functional factor V allele that maintains adequate factor V levels in blood (about 50%). However, the coinheritance of a factor V deficiency mutation on one allele and FV:Q506 on the other leads to a severe APC resistance phenotype similar to the homozygous FV:Q506 state.161-162
APC resistance and circumstantial risk factors for thrombosis
Pregnancy
During normal pregnancy the plasma concentrations of several of the proteins involved in the hemostatic mechanism change towards a hypercoagulable state. Although these changes are of physiologic importance in minimizing the risk of blood loss at delivery, they also increase the risk of thrombotic complications. In developed countries the overall incidence of thrombosis has been reported to be around 0.09% during pregnancy, with the risk being two to three-fold increased during puerperium.171 APC resistance appears to be an important predisposing risk factor for thrombosis in connection with pregnancy.68,172-176 In two Swedish studies, 45-60% of women with a history of pregnancy-related thrombosis were found to be APC resistant.172-173 Carriers of the FV:Q506 mutation appeared to be especially prone to developing thrombosis in early pregnancy and after delivery, compared to non-carriers of the mutation.173 APC resistance also seems to be associated with an increased risk of second trimester miscarriage related to placental infarction,177-178 and may be involved in the mechanism of preeclampsia.282
Elevated factor VIII levels
| Genotype | Non-user – | OCs (overall) | OCs (levonorgestrel) | OCs (desogestrel) |
|---|---|---|---|---|
| Normal | 1 | 4 | 4 | 9 |
| Heterozygotes | 8 | 32 | 15 | 48 |
| Homozygotes | 80 | 320 | 150 | 480 |
Oral contraceptives
The use of oral contraceptives (OCs) is a much-debated risk factor for thrombosis. Numerous studies have shown that oral contraceptives increase the risk of thrombosis about two to nine-fold depending on the active substance used, compared to non-users.185-186 However, it is still largely unclear why oral contraceptives cause thrombosis in some women. Similar to pregnancy, increased levels of procoagulant factors, as well as reduced APC ratios and reduced levels of antithrombin and free protein S have been reported in women using OCs.179,187-190 For the majority these procoagulant changes are negligible, although for a small number of women with a genetic or acquired predisposition for thrombosis, the added prothrombotic influence of OCs may be sufficient to trigger a thrombotic event.191
The risk of venous thrombosis in OC users with APC resistance has been investigated by Vandenbroucke et al.185 The risk of thrombosis among OC users in this study was shown to increase 4-fold when compared to women not using OCs (baseline risk about 0.01% annually). Women heterozygous for the FV:Q506 mutation who did not use OCs showed an 8-fold increase in thrombotic risk, whereas a 35-fold increase in risk was shown in OC users heterozygous for the mutation. Thus, the joint effect of the two risk factors appears to be multiplicative. Through a similar effect, the increase in risk for homozygotes using OCs is several 100-fold (Table 9).54,185 Clinical studies also demonstrate that OCs leads to an unacceptably high risk of venous thrombosis in females with homozygous FV:Q506.192
Hellgren et al investigated 28 women with a history of thrombosis in connection with OCs and they found that nine (32%) of the women had APC ratios <2.0, indicating APC resistance.172 None of the women investigated were using OCs at the time the blood samples were taken and in only one of nine patients with APC resistance was an additional risk factor for thrombosis identified.
Considering the high prevalence of APC resistance in the general population, a substantial number of women are placed at a higher risk by using OCs. In North America alone there are about 10 million OC users.193 Assuming the allele frequency for FV:Q506 is 0.025, then roughly 500,000 would be heterozygous and 6,000 homozygous for the mutation. Since the annual thrombotic risk is about 0.3% for heterozygotes and 3% for homozygotes using OCs, it can be calculated that around 2,000 thrombosis cases per year in North America are caused by the combination of APC resistance and OCs. This figure should be compared to the number of thrombosis cases in OC users without the mutation, which is about 4,000 cases per year. Thus, in the presence of both risk factors (OCs + mutation), venous thrombosis appears to develop in a substantial number of women who would never have had thrombosis in the presence of either risk factor alone. It can be estimated that 1-2% of all cases of venous thrombosis caused by OCs will have a fatal outcome due to pulmonary embolism.
Physicians who prescribe OCs generally interview the woman about her family history to learn whether there are any cases of thrombosis, indicating a genetic predisposition. However, since many homozygous APC resistance patients have asymptomatic parents that are heterozygous, it would not be suspected from interviewdata alone. This fact raises the question as to whether all women should be screened for the FV:Q506 mutation prior to prescribing OCs.190,194-197 The potential reduction in mortality and morbidity cases due to thromboembolism caused by the pill must, however, be weighed against the risk of thrombosis in connection with unwanted pregnancy.
Surgery
Patients undergoing major trauma or surgery, in particular orthopedic or abdominal surgery, generally run a high risk of experiencing thromboembolic events due to a sustained activation of coagulation (Table 10). The precise risk for an individual is determined not only by the type, extent and duration of the surgical trauma, but also by the accumulation of predisposing risk factors such as advanced age, morbidity and thrombophilia.199 The general and standardized use of heparin for post-operative thromboprophylaxis has reduced thrombotic complications to a great extent during recent decades. However, it still occurs in a substantial number of patients. Thus, it may be beneficial to pre-operatively identify these individuals at higher risk in order to adopt a more individualized and adequate prophylaxis.283 The latter approach is supported by recent studies, showing that prolonged prophylaxis using LMW heparin in connection with hip replacement resulted in a 50% reduction in venographically verified deep venous thrombosis.201-202 More studies are needed in this area to evaluate whether individualized prophylactic treatment might be beneficial in patients with genetic risk factors for thrombosis.
At present, the underlying role of APC resistance in postoperative thrombosis is under intense research. In a Danish study it was found that 30% of patients who developed deep venous thrombosis after knee arthroplasty were APC resistant.200 APC resistance was also shown to be a risk factor for arterial reocclusion of vascular grafts in peripheral vascular surgery.136,138 These studies are highly interesting and suggest that it would be cost-benefical to screen for APC resistance pre-operatively in order to reduce post-operative thrombosis.
Antiphospholipid antibodies
Lupus anticoagulant and anticardiolipin antibodies are acquired antiphosholipid antibodies (APAs) which were initially described in patients with systemic lupus erythematosus (SLE). Recently, the interest in these types of antibodies, which are more correctly described as antibodies directed against phospholipid-protein complexes, has focused mainly on their strong correlation with thrombotic disease, thrombocytopenia and recurrent fetal loss. The association of one of these complications and the presence of APAs has been termed the antiphospholipid syndrome.203 The mechanism underlying the syndrome is at present unknown, although various interferences in the protein C pathway have been suggested as being a possible pathogenic mechanism for thrombosis.204 One such interference may be the selective blocking of APC on phospholipid surfaces by specific types of APAs, leading to an acquired APC resistance.41,205-207 Several reports have also demonstrated acquired APC resistance to be a relatively common feature in patients with the antiphospholipid syndrome,206,208-211 even when the uncertainties of lowered APC ratios caused by APA-induced prolongation of the basal APTT were considered.206 At present, there is no clear clinical evidence to support a higher thrombotic risk among patients with the antiphospholipid syndrome and acquired APC resistance.210,214,215 Furthermore, the FV:Q506 mutation does not seem to be over-represented in patients with the syndrome compared with healthy controls.210-212
When to test for APC resistance phenotype and FV:Q506genotype
Up to now, the classic APC resistance test has been used mainly as a simple screening assay for the FV:Q506 mutation. However, several limitations have been reported for this application. Firstly, this test has a sensitivity and specificity for the mutation which is usually in the range 75-90%.219-220 Secondly, the test is only reliable if the basal APTT-reaction is within the normal range,221 which therefore disqualifies many APC-resistant patients on anticoagulant therapy from testing.222As the classic APC resistance test stands today, it is not recommended for FV:Q506 mutation screening. The classic test provides phenotypic information about APC resistance and should primarily be used as a complimentary test to the modified APC resistance test in a thrombosis investigation. A preliminary guideline for how to use the classic and modified APC resistance test is given in Table 11.
Table 11. Preliminary guideline for APC resistance and/or FV:Q506 testing.276
| 1. Caucasian patients with venous thrombosis should be tested, since the risk in APC resistant individuals is life-long. The identification of a defect is useful in decisions concerning anticoagulant therapy. |
| 2. First-degree relatives to thrombosis patients with inherited APC resistance should be tested as this may help in estimating the thrombotic risk profile of the family and the prevention of future thrombotic events. |
| 3. Thrombosis patients known to carry other genetic defects associated with thrombophilia should be tested for APC resistance, as symptomatic individuals tend to have more than one defect. |
| 4. FV:Q506 screening of patients with arterial thrombosis is not warranted except for young patients. |
| 5. General screening for FV:Q506 may prove to be beneficial before exposure to circumstantial risk factors such as prior to the prescription of oral contraceptives. |
APC resistance phenotyping
Interestingly, the APC ratio is not a simple, one-variable reflection of the APC response in vivo. Instead, it seems to reflect an anticoagulant system response that may decrease under a hypercoagulable state.139 A poor anticoagulant response to APC, independent of the FV:Q506 mutation, could therefore be a thrombotic risk factor or risk marker in a wide range of conditions and circumstances including venous thromboembolism,16,139 antiphospholipid protein syndrome,209 second trimester miscarriage,177 systemic sclerosis,170 ischemic stroke,140-141 occlusion after vascular surgery,138 pregnancy174 and the use of oral contraceptives.188-190 Future studies will clarify whether the phenotypic APC ratio obtained from the classic test may serve as a predictor of venous and arterial thrombotic events.
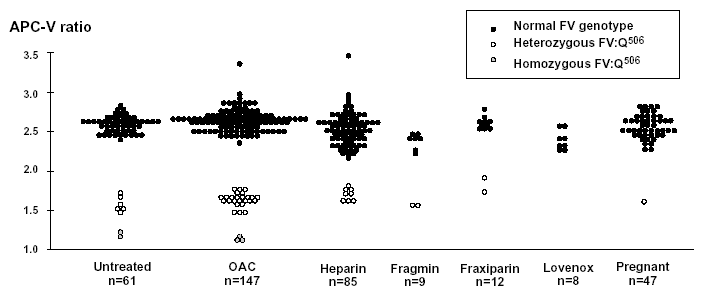
Figure 12. Relationship between APC-V ratios and the factor V:Q506 allele in various sample categories as determined using the Coatest® APC Resistance V method. Complete discrimination was obtained between the normal and mutated factor V genotypes. Instrument: ACL. OAC= oral anticoagulant therapy (from ref. 278 with extension of data for pregnant women).
Screening for FV:Q506
The modification of the APC resistance test, in which sample plasma is prediluted in an excess of stabilized factor V-deficient plasma, improves the discrimination for the FV:Q506 mutation dramatically.229-236 The modification also allows for the testing of patients on anticoagulant therapy and strongly reduces the influence of preanalytical variables such as storage and plasma handling. Evaluation of this test using different categories of clinical samples showed that the specificity and sensitivity for the presence of the FV:Q506 mutation was 100% (Figure 12).278 The robustness and simple format of the modified test, together with its high discrimination between normal and mutated factor V genotypes, makes it an ideal tool for FV:Q506 mutation screening. An important point when prediluting the sample plasma in factor V-deficient plasma is that phenotypic expressions related to factors other than factor V are lost. In order to obtain phenotypic and genotypic information for a correct thrombophilia diagnosis, one approach could be to analyze each plasma sample using both the classic and the modified APC resistance test.233 This strategy identifies APC-resistant patients, both with and without the FV:Q506 mutation, and keeps the need for confirmatory gene analysis to a minimum.
Management of APC resistant patients
A potentially large number of thrombosis-prone individuals with APC resistance will most likely be identified in the near future. This of course raises the question of patient management. At present there are no established guidelines for managing thrombotic patients with APC resistance, although it is generally agreed that they should be treated in the same way as the patients with antithrombin, protein S and protein C deficiency.249 An acute thrombotic episode should be treated conventionally with heparin for 5 to 10 days, followed by an oral anticoagulant (warfarin) within 24 hours to produce an International Normalized Ratio (INR) of 2.0 to 3.0. Patients should be given general advice on how to minimize the thrombotic risk, including dietary advice, cessation of smoking and avoiding long periods of immobility. Thrombophilic women should avoid oral contraceptives and all patients should be notified that they may require special treatment prior to surgical, medical or obstetric procedures that carry an increased thrombotic risk.
Anticoagulant treatments require individual considerations
Because only a proportion of the subjects with a heterozygous defect develop thrombosis, it is unjustifiable to put symptom-free APC resistant individuals on thromboprophylaxis solely on the basis of having a genetic defect. It is, however, essential that asymptomatic individuals in thrombosis-prone families are carefully counseled with respect to their defect and offered short-term prophylaxis in special situations where there is an extra risk of thrombosis. Women with a history of thrombosis and who have a known genetic defect may require anticoagulation throughout pregnancy, preferably by using dose-adjusted subcutaneous heparin.68 As a rule, all thrombophilic women should be offered thromboprophylaxis in conjunction with delivery and puerperium. Homozygous and heterozygous patients with a second anticoagulant defect should be given preventive therapy in all risk situations and long-term therapy should be considered if thrombosis is recurrent.
The prescription of oral contraceptives
When a woman has experienced venous thrombosis after oral contraceptive use, it is recommended that she is tested for the possible presence of the FV:Q506 mutation. If she is heterozygous for the mutation she should be carefully informed about her thrombotic risk and counseled about the type of contraceptive she should use in the future. The mere fact that she has had thrombosis indicates that the risk in her case is significant. If she is homozygous for the mutation, she should be strongly recommended to discontinue the use of oral contraceptives. As in any investigation of a young patient with venous thrombosis and APC resistance, it is also recommended to search for other causes of inherited thrombophilia.
Summary
The APC resistance phenotype is mainly diagnosed using the classic APTT-based test, which detects both inherited and acquired APC resistance. A modified APTT-based test using predilution of sample plasma with factor V deficiency plasma has been developed with high discrimination for factor V-related APC resistance. The factor V:Q506 mutation is highly prevalent among Caucasians (2-13%), it is present among people of Northern India (4%) and Saudi Arabia (2%), but is very rare among, for example Japanese, Eskimoes and Australian aborigines. The race-dependent distribution of the mutation may explain the higher incidence of thrombosis reported for Caucasians. The most common clinical manifestation of APC resistance is deep venous thrombosis, with or without pulmonary embolism. The relative risk of venous thrombosis for carriers of the factor V:Q506 mutation has been estimated to increase 8-fold for heterozygotes and 80-fold for homozygotes. The mutation does not seem to be an important risk factor for arterial thromboembolism, although acquired (or inherited) APC resistance not attributable to the mutation may be a risk factor for stroke as well as for venous thrombosis. The current recommendations for treating APC resistant patients are similar to those for other established inherited thrombophilic defects.
ORDER: 1-800-524-52224 | SUPPORT: 1-800-447-3846 | ABOUT US | CONTACT US | HOME
