APC RESISTANCE UNIVERSITY
Introduction | Biochemistry | Clinical Aspects | Key Reports | PubMed Abstracts | References | Assay Methods | APC Resistance Phenotyping | Factor V Mutation Screening | FAQ
ASSAY METHODS
Laboratory determination of APC resistance
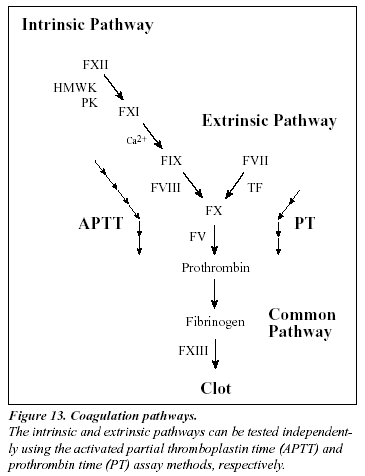
The laboratory analysis of poor anticoagulant response to APC is based on an activated partial thromboplastin time (APTT) assay, which is modified in the manner described by Dahlbäck et al.8 The standard APTT reaction begins by adding a surface-activating agent (e.g. kaolin, silica, ellagic acid) and a phospholipid preparation to citrated, platelet-poor plasma, thereby achieving maximum activation of factor XI. The plasma is then recalcified in order to activate the coagulation cascade and the time for clot formation is measured. The name APTT originates from the fact that the phospholipid reagents were originally derived from a lipid-enriched extract of complete thromboplastin (now called tissue factor), hence the term partial thromboplastin. The APTT reaction is dependent on factors of both the intrinsic and the common pathway of the coagulation system (Figure 13).
The classic APC resistance test
In the classic APC resistance test (available as Coatest® APC resistance), two APTT reactions are performed, one in the presence of a carefully defined amount of APC and the other in its absence. The result can be calculated either as a prolongation of the clotting time in presence of APC or as the ratio between the clotting times in the presence and absence of APC. Use of the APC ratio for expression of the results is preferred, mainly due to its lesser susceptibility to preanalytical sample handling variations.225
As for any APTT-based assay, it is important to follow a standardized procedure for blood sampling and storage. The APTT reaction without the addition of APC should be within the normal range (25-40 sec) in order to obtain valid APC ratios.221 This means that plasma from patients undergoing therapies causing deficiencies in clotting factors, for example treatment with warfarin or heparin, will not allow for reliable APC resistance analysis. Anticoagulant therapies must therefore, if possible, be discontinued for at least one week to allow re-establishment of the baseline prothrombin level. An alternative method in the case of heparin therapy is to neutralize heparin in the sample using a heparin antagonist.244
Instrument effects
The APC ratios obtained from the analysis, using the Coatest APC Resistance kit, of plasmas from healthy individuals on different coagulation instruments is typically within the range 2-5. The precise cut-off value for a diagnosis may vary slightly due to the instrumentation and the condition of the specific instrument.226-228 In general, coagulation instruments with a turbidimetric or photometric clot detection principle offer better discrimination than instruments using an electromechanical detection principle.225,227
The normal APC ratio range
Since the cut-off ratio for an abnormal APC response may vary slightly between laboratories and instruments, it is recommended that laboratories establish their own normal range. The normal range can be determined by measuring the APC ratios from at least 50 healthy non-APC resistant individuals in the age range 20-65 years and calculating the median APC ratio. The APC ratio cut-off is then obtained by multiplying the median value by either 0.75 (median above 3) or by 0.8 (median below 3). Alternatively, the cut-off can be calculated as the mean minus two standard deviations. If it is not possible to perform factor V genotyping in the determination of the 50 healthy non-APC resistant individuals, a practical approach is to exclude the lowest 10% and highest 10% of the APC ratios before calculating the APC cut-off ratio.
Normalized APC ratio
In addition to the instrument effect on the APC ratio, different sources of APTT reagents as well as different batches of the same APTT reagent may show varying sensitivity to APC.225,277,247 In order to compare results from different laboratories accurately it may be advantageous to present APC ratios after having normalized them against a pooled normal plasma, PNP (APC ratio/PNPAPC ratio).225,277 The properties of the PNP are important to consider as mixing studies of different plasmas show that a 10% contribution or more of APC-resistant plasma in the PNP greatly affects the normalized APC ratios obtained.221
Effect of plasma preparation and handling
To ensure reliable results it is necessary to obtain proper control of preanalytical variables. Thus, blood samples must be centrifuged thoroughly and the plasma removed carefully in order to minimize the number of platelets in the sample.240-243 The main reason for this being that freeze-thaw cycles reduce the APC ratios in the presence of platelets due to rupture of platelet membranes leading to the exposure of procoagulant phospholipids and platelet-bound factor V. As a rule, samples should be handled in the same way as the controls used to establish the normal range, i.e. “like should be compared to like”.234
A routine single centrifugation of the blood sample at 2,000 x g for 20 minutes will normally be adequate to obtain platelet-poor plasma (<1% normal platelet count).221 Centrifugation should take place at room temperature in order to avoid cold activation since this may eventually lead to the activation of factors VIII and V. Careful separation of plasma from platelets is achieved by leaving 0.5-1.0 cm of plasma above the cell layer. If plasma is to be frozen, it should be frozen rapidly in volumes of £1ml, at -20 °C or below.
Antiphospholipid antibodies
The presence of acquired antiphospholipid antibodies, such as lupus anticoagulant, is a relatively common finding in thrombophilic plasma. Because the antibodies react with anio
Influence of factors V and VIII
nic phospholipids they may give rise to a prolonged clotting time and thus may influence the APC ratio (decreased ratio).237 It has been observed that the effect of lupus anticoagulants can be surmounted by the addition of excess phospholipid, however further studies are needed to confirm this observation.239
Mixing experiments of different plasmas have demonstrated that changes in factor V levels between 12-100% do not have any significant effect on the APC ratio. 245 Very high factor VIII levels, for example in connection with pregnancy and inflammatory states, have been reported to lower the APC ratio,183-184,246 although the actual correlation between factor VIII activity and the APC ratio appears to be weak (Figure 11, page 21).
Influence of factors II, IX, X, protein S and protein C
Prothrombin and factor X concentrations below 50% tend to produce higher APC ratios. In contrast, the APC ratio is not influenced to any great extent by variations in factor IX and protein S concentrations down to 30% of normal.225 Variations in plasma levels of protein C have no influence on the APC ratio since a standardized amount of exogenous APC is added in the assay. On the other hand, it should be noted that the factor V:Q506 mutation may influence certain clotting-based functional protein S and protein C assays leading to the incorrect diagnosis of APC resistance as either protein S or protein C type II deficiency.250-251
Figure 14. Comparison of APC ratios obtained from the classic APC resistance test, with APC-V ratios obtained from the modified test method. Both these methods complement each other. The original method detects thrombosis-prone individuals with APC resistance due to the FV:Q506 mutation, unknown mutations or acquired factors and indicates the severity of their hypercoagulable state. The modified method provides a high discrimination for the factor V:Q506 mutation and allows for analysis of plasma from patients on oral anticoagulant or heparin therapy. The same individuals were tested in both groups. Instrument ACL.
The modified APC resistance test

A modification of the classic APC resistance test has been described in which the sample plasma is mixed with an excess of factor V-deficient plasma in the range 1:5 to 1:20.229-232,255 A predilution stage normalizes the concentration of plasma proteins involved in the formation and regulation of thrombin, except for factor V, resulting in an improved discrimination for the factor V:Q506 mutation (Figure 14).234-236 Because prediluted plasma samples obtained from patients undergoing oral anticoagulant therapy have an APTT reaction within the normal range independent of treatment intensity, it is now possible to test for APC resistance due to factor V mutation in a large group of patients previously disqualified from APC resistance testing. The inclusion of a heparin antagonist such as Polybrene® in the factor V deficiency plasma also makes it possible to analyze samples with heparin levels £ 1 IU/ml (unfractionated and LMW preparations).278
The use of a stabilized factor V-deficient prediluent (V-DEF Plasma) in the modified test, strongly reduces the influence of pre-analytical variations such as plasma handling and storage. Furthermore, no significant difference between fresh and frozen plasma is obtained and platelet counts up to 15,000/mL are easily tolerated.279 Predilution (1+4) reduces most sources of interference, although it cannot be excluded that the analysis of plasma from patients with antiphospholipid antibodies may result in an abnormal APTT. In such cases, increasing the dilution factor may correct the test result (e.g. 1+9 or 1+19). In neonates and infants (< 6 months of age) a predilution factor of 1+9 is needed because of their special hemostatic condition.248
Alternative functional assays for APC resistance
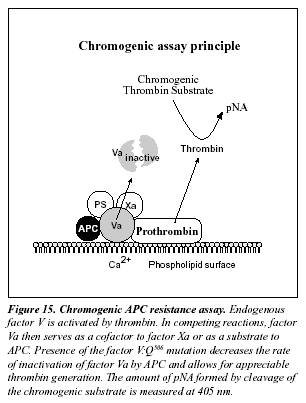
The present detailed understanding of the mechanism behind APC resistance has produced a number of alternative assay concepts for APC resistance (Table 11).252-259 A novel chromogenic APC resistance assay is presented in Figure 15.280 The assay is based on the addition of factor Xa to diluted test plasma in the presence of calcium ions, phospholipids and APC. Presence of the factor V:Q506 mutation decreases the rate of inactivation of factor Va by APC and allows for appreciable thrombin generation. This is measured by the hydrolysis of a chromogenic thrombin substrate. Preliminary results show complete concordance between the chromogenic assay and the modified APC resistance test.280
PCR-based assays for FV:Q506
Identification of a mutation is generally accomplished by amplifying genomic material containing the mutation, followed by a mutation detection procedure. Amplification is achieved by the polymerase chain reaction (PCR) technique using either DNA or mRNA as a template.274-275 The key components in a standard PCR include a pair of oligonucleotide primers (around 20 bases long), the DNA building blocks; deoxyribonucleoside triphosphates, and a heat-resistant DNA polymerase (e.g. Taq). These components are added to a closed test tube containing the template DNA. A temperature-driven cyclic process is then initiated, consisting of three stages: 1) DNA strand separation, 2) Annealing of primers, and 3) DNA synthesis (Figure 16). A central point of the PCR is that all new DNA strands serve as templates in successive cycles. The new DNA, consisting of the target sequence flanked by primers, increases exponentially in subsequent cycles. After n cycles the DNA target sequence is amplified 2nfold. The amplification is a million-fold after 20 cycles and can be carried out in less than an hour. In the seminal work by Bertina el al,13 in which the FV:Q506 mutation was first identified, the detection stage was performed by enzymatic digestion of the amplification product using the Mnl I restriction enzyme, followed by agarose gel electrophoresis. Presence of the mutation removes one of normally two cleavage sites for Mnl I. The resulting number and size of the fragments indicates whether the mutation is present or not.
Apart from methods involving direct sequencing of cDNA,14 a vast number of PCR-based methods for the determination of the factor V:Q506 mutation have been published. These involve improvements of the original method,262 , chemiluminescent detection,261 whole blood PCR,260 two-stage PCR using restriction enzymes Mnl I and Nla III,264 use of allele-specific probes,263,269,271 with the introduction of a Taq 1,267,270 or a Hind III recognition site,272 in combination with Elisa,265 or with capillary electrophoresis.266 A PCR assay using allele-specific primers after microwave irradiation of leukocytes is a novel method which eliminates the need to extract the genomic DNA.268 The determination of FV:Q506 can also be achieved by using the direct RNA amplification technique (NASBA), together with the detection procedure ELGA (enzyme-linked gel assay).273

Summary
The laboratory determination of APC resistance is based on a clotting assay that measures two APTT-reactions, one performed in the presence of exogenous APC and the other in its absence. To obtain reliable results with the classic APC resistance test method, it is necessary for the basal APTT reaction to be within the normal range (25-40 sec). If results from different laboratories are to be compared, it may be beneficial to normalize the APC ratio against the APC ratio of a normal plasma pool. A modification of the classic test is now available, which involves a 1+4 predilution of samples with factor V deficient plasma. This assay variant not only gives a 100% discrimination for the FV:Q506 mutation, but also strongly reduces the influence of preanalytical variations such as plasma handling and storage. Since predilution in factor V-deficient plasma normalizes the basal APTT it safely allows for testing of patients on anticoagulant therapies. DNA-based methods for the detection of the FV:Q506 mutation include primarily PCR amplification of the defect region from genomic material followed by the restriction enzyme cleavage.
ORDER: 1-800-524-52224 | SUPPORT: 1-800-447-3846 | ABOUT US | CONTACT US | HOME